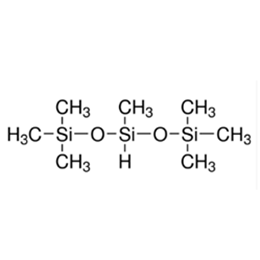
Silicone surfactants are a class of surfactants with polydimethylsiloxane as their hydrophobic backbone and one or more organosilicon polar groups attached to their intermediate or end positions. The Si-O bond energy of the hydrophobic group in its structure is higher than the C-C and C-O bond energies of traditional carbon chain surfactants, which makes it more hydrophobic and stable; its large molecular weight and multi-branched structure make it have excellent low-temperature performance and compatibility, and it is an efficient surfactant.
![]()
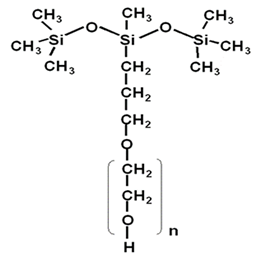
The general formula of its structure is:
![]()
By changing the m, n and R groups in the formula, silicone surfactants with different molar masses (viscosity) and hydrophilic-lipophilic balance values (HLB values) can be produced for various applications, such as foam leveling agents for polyurethane foams, emulsifiers, personal care materials, leveling agents for coatings, defoamers, antistatic agents, plastic modifiers, wetting agents, fuel additives, fabric finishing agents, water-soluble lubricants, mold release agents, pesticide additives, agricultural adjuvants etc.
01 Silicone surfactant features
1. Excellent performance in reducing surface tension.
2. Excellent wetting performance.
3. Antifoaming property and foam stabilization property.
4. Toxicity is basically physiologically inert.
5. Great emulsifying effect and good matching performance.
02 How to evaluate the effect of silicone surfactants?
Critical micelle concentration (CMC) is a parameter used to determine the minimum required to reduce maximum water surface tension. The micelles formed above the CMC separate the hydrophobic contaminants from the soil and hold the micelles within their hydrophobic cores, thereby recycling them into the aqueous phase. the CMC value determines the efficiency of any biosurfactant; therefore, this value is critical for biosurfactant comparisons.
03 Classification of silicone surfactants
Silicone surfactants can be divided into four categories: nonionic, anionic, cationic and zwitterionic according to the chemical properties of the hydrophilic group R in their chemical structure. Among them, nonionic surfactants are the most studied, The most widely used.
1. Cationic silicone surfactant
If the R group contains structural units such as alkyl quaternary ammonium compounds, amido quaternary ammonium compounds and imidazoline derivative quaternary ammonium compounds, it is called a cationic organosilicon surfactant. Among the cationic surfactants, the cationic polysiloxane quaternary ammonium salt surfactant is the most widely used. The cationic polysiloxane quaternary ammonium salt surfactant has a large molar mass, can be compatible with anionic surfactants, is non-irritating to human skin and eyes, and has a certain antibacterial ability. Stablize. The macromolecules of this product contain hydrophobic long-chain polysiloxane chains, which give it excellent smoothness and softness.
2. Anionic silicone surfactant
When the R group contains structural units such as phosphate ester salt, sulfate salt, carboxylate salt, sulfonate salt and sulfosuccinamide ester, it is called an anionic silicone surfactant. When R is the structure shown in the figure below, it is called an anionic polysiloxane phosphate salt surfactant.
![]()
When R' is a fatty acid functional group, it is a polysiloxane phosphobetaine amphoteric surfactant. The surfactant molecule has both the structure and properties of phosphobetaine and the structure and properties of polysiloxane. If a low molar mass polysilane is selected, the polysiloxane characteristic is weak; on the contrary, if a polysiloxane with a large molar mass is selected, the polysiloxane characteristic is remarkable. Such silicone polymers products have the characteristics of low toxicity, antibacterial, hard water resistance, and good compatibility with various surfactants.
3. Nonionic silicone surfactants
When the R group contains units such as polyether, alkanolamide, ester, and glycoside, it is a nonionic surfactant. Among them, polyether silicone surfactants are the most used, such as:
![]()
Non-ionic polyether silicone surfactant is composed of polysiloxane segment (A) and polyether segment (B). The combination methods are: AB type, ABA type, BAB type, and (AB) Types such as n-type, branched-chain and side-chain type. The connection between the polyether segment and the siloxane segment has two ways, namely Si-O-C type and Si-C type. The former is unstable and belongs to the hydrolysis type; the latter is stable to water and is called the non-hydrolysis type.
4、Amphoteric silicone surfactants
If the R group contains a structure such as phosphate betaine or betaine, it is called amphoteric polysiloxane surfactant.
04 Methods for the synthesis of silicone surfactants
1. Synthesis of cationic silicone surfactants
The reaction is carried out in an inert solvent such as benzene, acetone, carbon tetrachloride, xylene, and toluene.
![]()
2. Synthesis of anionic silicone surfactants
![]()
3. Synthesis of nonionic silicone surfactants
From the method of copolymerization preparation and from hydrolysis and chemical stability, it can be divided into two types of synthesis methods: Copolymers linked by Si-O-C chains and copolymers linked by Si-C chains. The synthesis can be divided into two steps: The first step is to synthesize polysiloxane, and the second step is to make polysiloxane form block copolymers with polyoxoalkane in the form of Si—O—C/Si—C.
05 Performance of silicone surfactants
1. Interfacial properties of organosilicon surfactants
As the main chain of silicone surfactants is a soft Si-O bond, it is neither hydrophilic nor lipophilic, so it can be used in the aqueous solution and non-aqueous media where ordinary hydrocarbon surfactants cannot be applied. On the other hand, silicone surfactants arranged at the interface with methylene groups can reduce the surface tension to about 20 mN/m, whereas ordinary hydrocarbon surfactants arranged at the interface with methylene groups can only reduce the surface tension to about 30 mN/m.
The most used silicone surfactant is the EO/PO modified silicone surfactant, whose performance is related to a variety of factors such as the ratio of EO/PO and the degree of polymerization of the surfactant; EO is the hydrophilic part of the surfactant modification group and PO is the lipophilic part. When the ratio of EO/PO changes, the performance of the silicone surfactant will change.
It is found that if the EO/PO ratio becomes larger, the HLB value of the surfactant will increase, indicating enhanced hydrophilicity; a smaller EO/PO ratio will make the surfactant less hydrophilic. When grafted polyether-modified silicone oils have the same length of EO, their surface tension increases as the degree of polysiloxane polymerization decreases. This is due to the fact that the shorter the molecular chain of the polysiloxane, the tighter the build-up at the air/water interface and the more methyl groups on the surface. When PO is introduced, it increases the hydrophobicity of the silicone polyethers' chain, thus increasing the surface tension of the polyether-modified silicone oil.
2. Superwettability of silicone surfactants
Trisiloxane surfactants can not only reduce the interfacial tension at the oil/water interface, but may also wettability expand on low-energy hydrophobic surfaces, an ability known as "super-wettability" or "super-spreadability". This phenomenon is thought to be due to the presence of specific surfactant aggregates in the solution. Therefore, silicone surfactants can be used as wetting agent.
The reason why polydimethylsiloxane chains spread easily on polar surfaces (e.g. water, metals, fibres, etc.) is that the oxygen in the silicone chain can form oxygen bonds with polar molecules or groups of atoms, increasing the force between the silicone chain and the polar surface molecules and causing them to spread into a single molecular layer, thus causing the hydrophobic silicone to lie across the polar surface in a characteristic "stretched chain This results in the hydrophobic silicone lying across the polar surface in a characteristic "stretched chain" configuration, whereas the hydrophobic groups of ordinary surfactants lie upright on the polar surface.
When the methyl group in a polysiloxane is replaced by other groups (e.g. large alkyl, alicyclic, aryl, silicon-functional or carbon-functional groups), this inevitably affects the hydrophobicity of the polysiloxane and the rate and state of spreading on polar surfaces due to the change in the polarity or spatial site resistance of the substituent. The number and distribution of substituents on the silicone chain can have the same effect. For example, the substitution of methyl groups by larger alkyl or aryl groups significantly reduces the spreading ability of the polysiloxane, as well as its ability to orient itself on polar surfaces.
3. The ability of silicone surfactants to stabilize emulsions
Some grafted silicone surfactants maintain emulsion stability in the presence of salts, ethanol, and organic solvents, an ability that traditional hydrocarbon surfactants do not have. The interaction force at the interface of silicon surfactant was found by atomic microscopy (AFM). Nonionic surfactants lose surface activity in 25% ethanol solution, while silicone surfactants can still reduce surface tension when the volume fraction of ethanol reaches 80%. This property of silicone surfactants reflects that polydimethylsiloxane is not only hydrophobic, but also insoluble in organic solvents with increasing polydimethylsiloxane content.
4. The role of silicone surfactants and CO2
Rocha et al. believed that polyoxyethylene ether trisiloxane surfactants can make CO2 and water form emulsions. By adjusting the number of EO and changing the "hydrophilic and CO2-philic balance (HCB) value" of the surfactants, the emulsions can be made of CO2 Water (W/C) converted to CO2 in water (C/W). Sarbu et al. believe that the properties of polysiloxane surfactants enable them to be used in supercritical CO2. Fink et al. considered the phase behavior of silicone surfactants in supercritical CO2, and found that the phase behavior was very sensitive to CO2-phobic groups, but less sensitive to the size of siloxane, and found a random liquid crystal phase. Folk et al. prepared a series of cationic silicone surfactants and studied their behavior in high-density CO2. Changing the chain length and counterion of polydimethylsiloxane strongly changes its surface activity in critical CO2.
06 Application of silicone surfactants
1. Personal care and cosmetic products
Because silicone surfactants have many advantages such as non-toxicity, non-skin irritation, anti-oxidation, UV protection, good biocompatibility, and excellent waterproof and breathable properties, they are widely used in cosmetics, shampoo and hair care products, creams, etc. Class products and products have a certain degree of application. Because of its low surface tension and suitable viscosity, silicone surfactant enhanced spreading of cosmetics on the skin and hair surface, improve the moisturizing and retention of cosmetics on the skin, and maintain the normal ventilation of the skin. Hair is shiny, manageable, smooth, antistatic and soft.
![]()
2. Textile industry
Silicone surfactants have antistatic properties, softness, and good sterilization and disinfection capabilities, and can impart a good softening effect to fibers. Cationic silicone surfactants are mainly used as antistatic agents and softeners in the textile industry. Quaternary ammonium salt cationic silicone surfactants are used in the hygienic finishing of fiber products, and have good sterilization and mildew resistance. And safe and durable.
![]()
3. Pesticides
In pesticide processing, adding 0.05% - 0.1% (mass fraction) of organosilicon surfactant as an auxiliary agent can optimize the physical properties and chemical stability of the preparation, increase the variety of preparations, and expand the scope of application. The leaf and stem epidermis of plants often have anti-wetting components or structures, and often have negative charges, which have a repulsive effect on pesticide liquids, and the addition of silicone surfactants can promote the smooth attachment of pesticide formulations to plants, Maintenance, spreading and penetration play a key role in improving the efficacy of the drug. Silicone surfactants can reduce their surface tension, allowing water to penetrate into the trachea of insects, making them lethal, as insecticide adjuvants.
- XJY-703 Bis (trimethylsiloxy) methylsiloxane/Heptamethyltrisiloxane contains a highly reactive silicon-hydrogen bond and it is a basic material for synthetic Polyaleneoxide Modified Heptamentyltrisiloxane.
- XJY-207 Polyalkyleneoxide Modified Heptamethyltrisiloxane is a modified trisiloxane silicone surfactant with a special structure. It has super properties of wetness and expansion as well as stomatal penetration rate which normal surfactants cannot meet. XJY-207 can improve the prevention effect of pesticides and reduce the spray volume. It is used in the fields of pesticides herbicides, insecticides, acaricides, fungicides, plant growth regulating agents, and other aspects.
![]()
4. Food and medicine
Silicone defoamer is formulated with modified polysiloxane as the main component, adding a variety of non-ionic surfactants, etc. It has excellent defoaming and foam control performance, less dosage, convenient use, and non-toxic, non-toxic corrosive. And it has good defoaming and anti-foaming effects on the foam produced in the production of monosodium glutamate, soy products and antibiotics.
- XJY-706 1,1,3,3-Tetramethyldisiloxane can be used as medicine intermedia
![]()
5. Leather chemicals
Silicone surfactants are mainly used as fatliquors and softeners in leather because of their good lubricating properties and water repellency. The organosilicon fatliquoring agent prepared by the graft copolymerization of it and the grease solves the problem that the silicon-containing surfactant is easy to migrate outward, and reduces the amount of the fatliquor and the cost. The leather fiber impregnated with silicone surfactant has good dispersion and lubricating properties, and the finished leather is soft, especially suitable for garment leather and shoe upper leather, and can also be used for fur manufacturing. Aminopolyether co-modified silicone surfactant is used as a fatliquoring agent, which can make the softness and hydrophilicity reach a satisfactory level. Bright and oily.
![]()
6. Machining
In the process of production, use and maintenance of metal products, it is necessary to clean all kinds of dirt (such as metal chips, cutting fluid, abrasives and various greases, dust and acids, alkalis, salts, etc. electrolytes and hand sweat, etc.) to ensure product internal and surface quality and prolong service life. Silicone surfactant cleaning agent has good cleaning performance and strong detergency. It can not only remove oil stains on metal surfaces, but also clean dirt such as hand sweat and inorganic salts. In addition, it is non-flammable, non-toxic and safe to use. As well as good corrosion inhibition and anti-corrosion ability, it can also save energy and reduce environmental pollution, and it is suitable for mechanized automatic cleaning.
![]()
7. Plastic industry
Silicone surfactant plays an important role in the production process of polyurethane foam, such as system dispersion, bubble growth, bubble stabilization and air chamber opening, etc. It is a good foam stabilizer and stabilizer for polyurethane foam. Using its surface activity, it can manufacture polyurethane soft foam, rigid foam, semi-rigid foam and high resilience foam. In addition, the application of flame-retardant silicone foam stabilizer in polyurethane foam is also more and more extensive.
![]()
07 Conclusion
Silicone surfactants have the advantages of cheap and easy-to-obtain raw materials, mild and easy-to-control processes, wide application, and large demand. They have good field performance, rapid development, and large market potential. The research on silicone surfactants by domestic scholars is making great strides to catch up with the international level, and some frontier research results have been achieved in the direction of a structural twinning degree of research. At the same time, there has also been more progress in the research on the cleaning and greening processes and products.
References:
"Sweet Silicones": Biocatalytic Reactions to Form Organosilicon Carbohydrate Macromers. Org. Lett. 2005; 7 :3857–3860.
Henkensmeier D, Abele BC, Candussio A, Thiem J (2004) Synthesis and characterization of terminal carbohydrate modified poly(dimethylsiloxane)s.
Wang G, Qu W, Du Z, Cao Q, Li Q (2011) Adsorption and aggregation behavior of tetrasiloxane-tailed surfactants containing oligo(ethylene oxide) methyl ether and a sugar moiety.
Kickelbick G, Bauer J, Husing N, Andersson M, Palmqvist A (2003) Spontaneous vesicle formation of short-chain amphiphilic polysiloxane-b-poly(ethylene oxide) block copolymers.
Soni SS, Sastry NV, George J, Bohidar HB (2003) Dynamic light scattering and viscosity studies on the association behavior of silicone surfactants in aqueous solutions.